In near-Earth space there are three places to mine. The first is the Earth itself. Some of the challenges to mining only on the Earth are (1) too much mining on Earth messes up habitats for life, (2) some of the best resources on Earth are already becoming scarce so their costs are rising, and (3) if we want to use Earth’s resources for anything we are doing in space then we have to launch them into space, and that is very expensive!
The second place to mine in near-Earth space is on a Near-Earth Asteroid (NEA) that we will bring back to orbit the Moon. We hope to start doing that very soon! This has been discussed in some of the prior posts.
The third place near the Earth where we can mine is the Moon. Unlike NEAs it’s always nearby, and the space mining enthusiasts (including myself) will tell you it is loaded with resources. Others will scoff at this idea and tell you that the transportation costs are so high that even if the Moon were made of pure gold it would be unprofitable to bring any back to Earth.

There is an ancient proverb that tells of a simpleton who, seeing the Moon’s reflection in a pond and thinking it was a floating cheese, reached for it and fell into the water. And there is a true tale from the 1700’s telling how the people of Wiltshire, England became known as the Moonrakers after they were caught raking a pond at night to retrieve smugglers’ contraband that had been hidden under water. They did some quick thinking and fooled the law enforcement authorities by acting as the proverbial simpletons, raking at the reflected image of the Moon because (they said) they thought it was a floating cheese.
So what should people think about us space miners who want to rake the actual Moon for its resources, as though it really were a big “cheese” floating in the pond of the sky? Are we the proverbial simpletons embodied, raking at an illusion until eventually we fall in like fools? Or are we like the Moonrakers of Wiltshire, getting ready to pull out the hidden goods and have the last laugh?
One Giant Ore Body
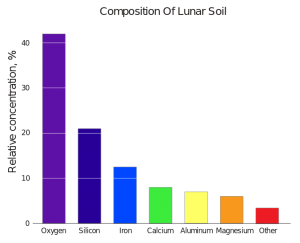
When I first joined the resource utilization community some years ago I was told there are no veins of gold on the Moon; no concentrated platinum deposits; no special locations of anything worth mining. (How that view has changed! More on that in a later post…) I was also told, however, that there is one very valuable resource that is available literally all over the Moon, everywhere you go. It is oxygen. The minerals of the Moon are more than 40% oxygen by mass. That is similar to the ordinary beach sand here on Cocoa Beach, for example. The grains of sand on the beach are composed of the mineral quartz, a crystalline structure of silicon dioxide — two oxygen atoms for every silicon atom. Oxygen has an atomic weight of 16, and silicon is 28, so oxygen comprises (16+16)/(16+16+28) = 53% of the mass of the sand on Cocoa Beach. The beach is more than half oxygen everywhere under your feet, and the Moon is very much like that. To get oxygen from the Moon you don’t need to seek out special veins or concentrations of it, special “ore bodies” in the lunar mountains or deep down in the crust. The whole Moon is one giant ore body of oxygen, one great big cheese. The cheese of lunar resources is oxygen. All you have to do is rake up the soil and use a simple chemical reaction to get the oxygen atoms out from the minerals. This is worth doing, because oxygen just happens to be the most valuable commodity in space when you want to conduct exploration missions!
The Most Expensive Part of Space Flight
Why is oxygen so valuable for conduction space exploration missions? Well, if you don’t plan to use the resources of space, then you have to launch everything from the Earth, and the most expensive single part of space missions is providing the rockets and the human labor to launch oxygen from the Earth, because oxygen is so heavy and we need so much of it for a mission. For example, consider the weight of propellants in the Space Shuttle’s big orange external tank. It contained hydrogen and oxygen, and they burned together to produce H2O – water! The Space Shuttle launched into space on a flame of water! So how much of the weight in that gigantic external tank was due to the hydrogen and how much was due to the oxygen? Oxygen has a molecular weight of 16 and hydrogen has a molecular weight of 1. Two hydrogens combine with one oxygen to make water, so that means 16/(16+1+1) = 89% of the mass in the tank was oxygen! And the propellants in the tank were roughly half the mass of the entire Space Shuttle at liftoff, so it turns out the overall mass of oxygen was about 38% of the total mass of the Space Shuttle and its cargo at liftoff. Now that’s the oxygen that it takes to get into orbit. If you have another rocket designed to go from Earth orbit all the way out to Mars, then similarly high amounts of oxygen must be used, and all that oxygen needs to be launched from Earth at high expense. Then, if you want to actually land on Mars, you need even more propellants to run the engines in the Mars lander, and you need even more propellants just to send the lander’s propellants from Earth out to Mars. And if you want to launch back off from Mars at the end of the mission, then you need even more propellants, and you need even more than that just to land the propellants on Mars, and you need even more propellants to send all the above propellants from Earth out to Mars. And if you want to come back from Mars to Earth, then you need even more. It all adds up: oxygen being used to deliver oxygen being used to deliver oxygen, and it all has to be launched from Earth at great expense. But if you play the Moonraker and get some of the oxygen “cheese” for a Mars mission from lunar soil (and get more of it from Martian soil), then the studies show you can cut the cost of a Mars mission by a factor of 3 or maybe even by a factor of 5. Oxygen derived from minerals in space truly is a big cheese.
Dust to Thrust

This is something we really can do! The technology to rake the Moon and get oxygen atoms out of the minerals is fairly mature. The space resources community has developed several different chemical reactions to make it possible and has built several prototype chemical reactors and taken them to a volcano in Hawaii for rigorous field testing. I was there in 2010 on Mauna Kea when one of these systems was tested. The site was chosen because it is very lunar-like with rugged terrain, volcanic minerals somewhat like those in the lunar soil, and lots of dust. We had robotic excavators raking up the dust, a regolith conveyance system moving the dust into a chemical reactor, solar concentrators collecting sunlight to melt the dust, a chemical reactor extracting oxygen from the melted dust, an oxygen liquefaction system to store the oxygen after it was extracted, and a small rocket thruster to burn the oxygen with methane fuel. Therefore, the system took volcanic dust and converted it into rocket thrust: “Dust-to-Thrust”! And it all worked.
So there you have it! The big cheese of lunar resources isn’t just an illusion. The Moon is a giant ore body providing a resource we need to accomplish Mars missions, and we have the knowledge and technologies to go up there get it. We’re not simpletons raking at a reflection, we’re going to be like the real-life Moonrakers of Wiltshire. And like them, we’re going to have the last laugh!
In the next post I will discuss another highly valuable resource on the Moon.

